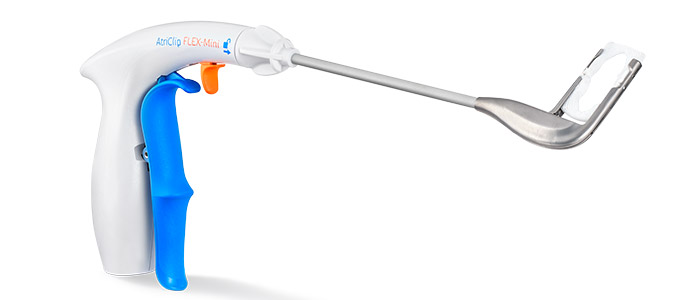
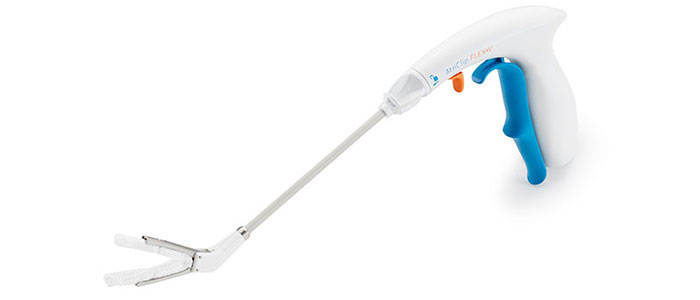
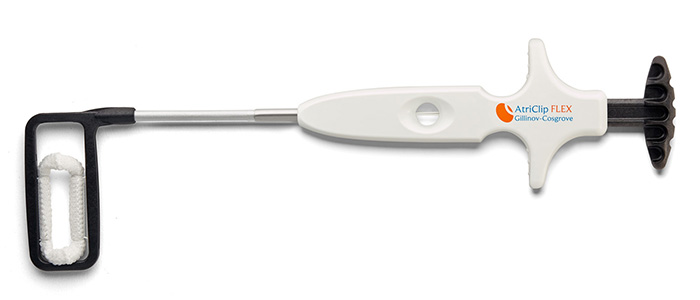
AtriClip Device: The Most Widely Implanted LAAM Device Worldwide
The AtriClip devices are epicardially applied to the base of the left atrial appendage (LAA) during cardiac surgery procedures such that the LAA is permanently excluded from the left atrium of the heart. This exclusion eliminates blood flow and electrical communication of blood between the left atrium (LA) and the LAA and results in the electrical isolation of the LAA from the rest of the heart.1-4
AtriClip Device Features
Epicardial Exclusion
- Implant is not in the blood stream
- Ischemic injury electrically isolates the LAA
- Atrophy and absorption of the LAA
Continuous Dynamic Closing Pressure
- Continuous closing force maintains LAA exclusion throughout changes to the tissue caused by ischemia.
Parallel / Linear Closing
- Minimized occurrence of tissue folds with optimal apposition of tissue along long axis of LAA ostium
Tissue Compression / Atraumatic
- No cutting, non-piercing, and atraumatic compression reduces risk of tissue tearing and bleeding
Types of AtriClip Devices
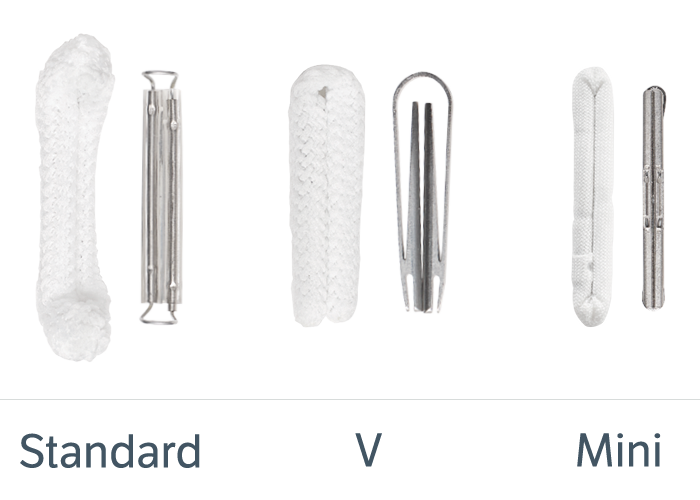
Devices are shown without fabric for informational purposes only.
Designed for Open Concomitant Surgery
Designed for Minimally Invasive Concomitant Surgery
The AtriClip devices are available on a variety of delivery systems optimized for open concomitant procedures as well as minimally invasive surgery, both through a thoracotomy and a port.
Each device is packaged with a Selection Guide that allows for determining the right size AtriClip device.
LAA Exclusion Upgraded to Class IA Recommendation
In the latest release of the ACC/AHA/ACCP/HRS Guidelines for diagnosis and management of atrial fibrillation, LAA management was upgraded to the highest recommendation. Over the past two decades, AtriCure has worked tirelessly to develop a portfolio of AtriClip devices that permanently exclude the LAA. Now with a Class IA recommendation, AtriCure is more determined than ever to advocate for the value and importance of LAA exclusion.
PM-US-1512C-1026-G
PM-US-1511D-1026-G
Development of the AtriClip Device
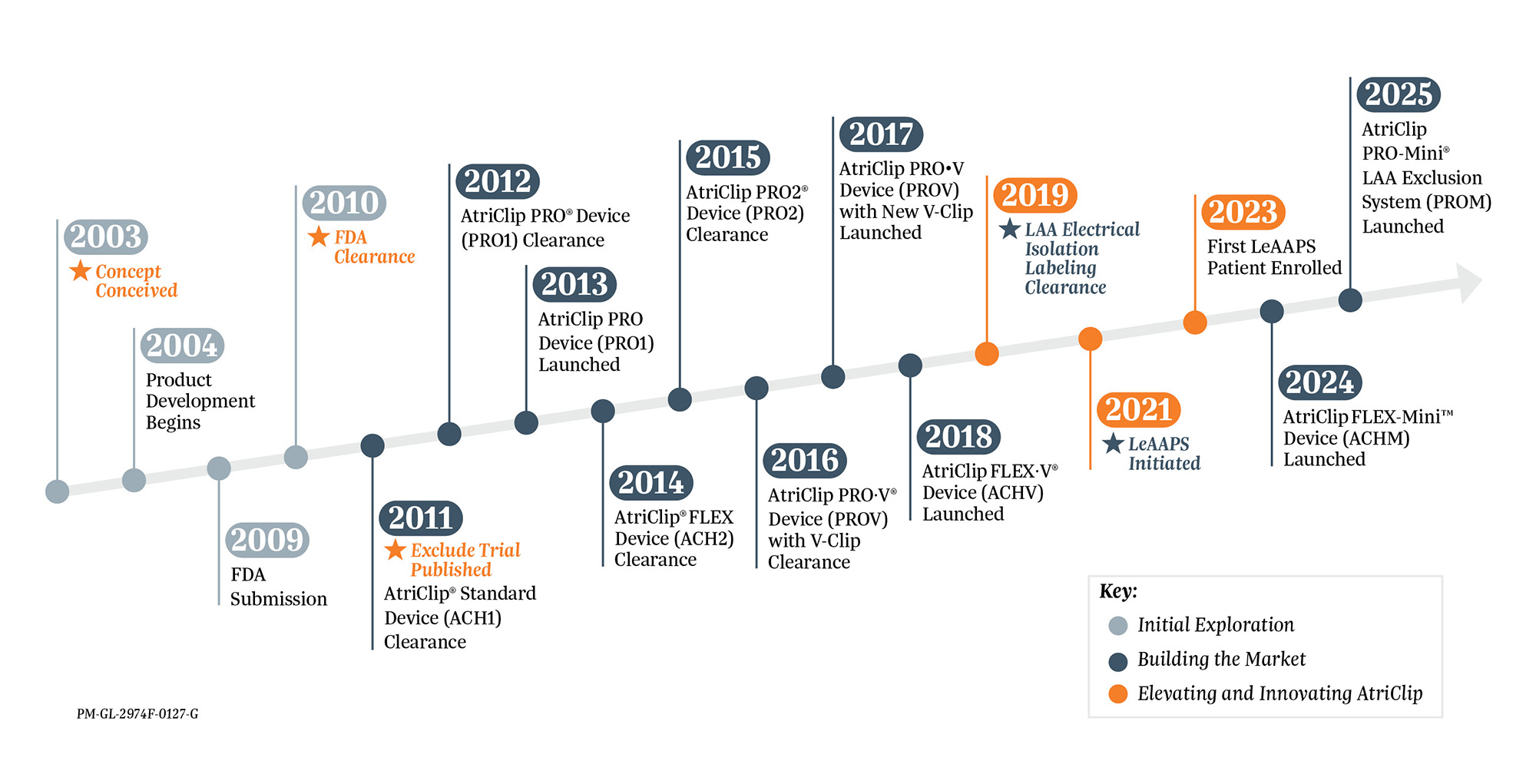
- Benussi, S. et al, Thoracoscopic Appendage Exclusion With an AtriClip Device As a Solo Treatment for Focal Atrial Tachycardia. Circulation, 2011, 123:1575-8.
- Starck, CT et al, Epicardial left atrial appendage clip occlusion also provides the electrical isolation of the left atrial appendage. Interactive Cardiovascular and Thoracic Surgery, 2012, 15(3):416-419.
- Kamohara K et al. A novel device for left atrial appendage exclusion. J Thorac Cardiovasc Surg 2005, 130(6):1639-44.
- Kamohara K et al, Evaluation of a novel device for left atrial appendage exclusion: The second-generation atrial exclusion device, J Thorac Cardiovasc Surg, 2006, 132:340-6.
Warning: The safety and effectiveness of this device in atrial rhythm control management, either alone or in combination with ablative treatment, has not been established.
The safety and effectiveness of this device for stroke prevention, either alone or in combination with cardiac surgery, has not been established.
- *AtriClip PRO•V Device is not included.