650,000
Patients served with ablation worldwide
The FIRST and ONLY Approved Surgical Device to Treat Atrial Fibrillation
| Features | OSL2 | OLL2 |
| Jaw Length | 53 mm | 65mm |
| Generator | ASU/ASB, MAG | ASU/ASB, MAG |

The Isolator Synergy clamp is the first and only surgical ablation device offered with FDA approval for the treatment of Atrial Fibrillation (Afib) and the only device, either catheter or surgical, to be approved for the treatment of persistent and long-standing persistent Afib.

Uses a dynamic monitoring algorithm that measures the tissue’s response to radiofrequency delivery 50 times per second, which results in a custom-made column-shaped lesion specific to a tissue’s length, width and composition.
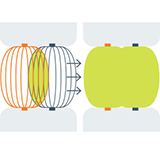
Stainless steel shaft and jaws maintain consistent tissue pressure and precise electrode alignment across entire length of the jaws. The Synergy clamp uses two pairs of electrodes, creating a more robust lesion.
See how the parallel jaw closure combined with dual electrodes and continuous monitoring create a uniform lesion.
Patients served with ablation worldwide
Surgical ablation expertise and leadership
Recommendation by the Society of Thoracic Surgeons for performing concomitant surgical ablation during open heart surgery to fix a coronary or valve